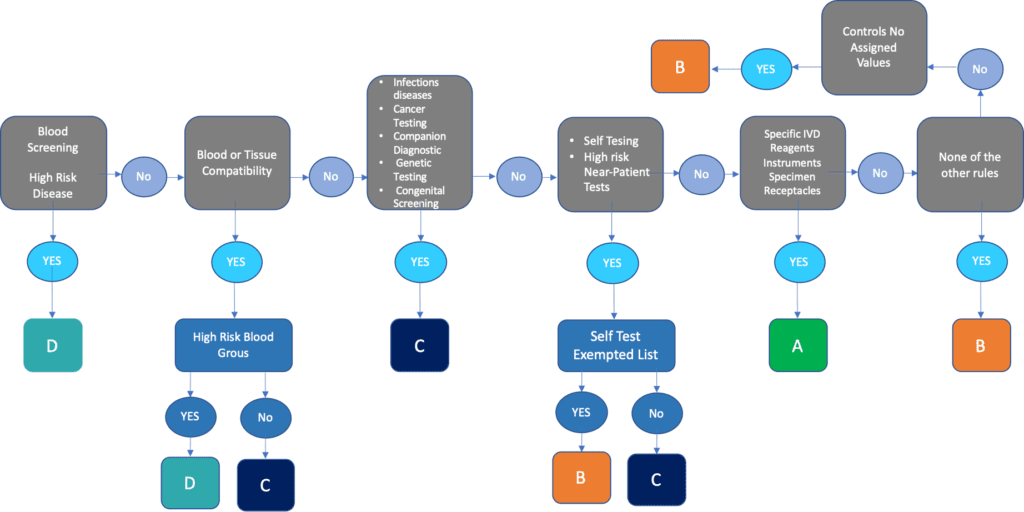
Class D In Vitro Diagnostic Medical Devices . this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. this guidance document is one of a series that together describe a global regulatory model for medical devices. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). Ponsibility of the manufacturer to ensure its products are in complia. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. what are the major ivdr changes?
from 4easyreg.com
questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. Ponsibility of the manufacturer to ensure its products are in complia. what are the major ivdr changes? this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance document is one of a series that together describe a global regulatory model for medical devices.
The New IVDR Classification for InVitro Diagnostic Devices
Class D In Vitro Diagnostic Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. Ponsibility of the manufacturer to ensure its products are in complia. what are the major ivdr changes? this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. this guidance document is one of a series that together describe a global regulatory model for medical devices. (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised.
From www.nagase.eu
Leading in In Vitro Diagnostics Solutions Nagase Europa GmbH Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). (1) for certain class d in. Class D In Vitro Diagnostic Medical Devices.
From apacmed.org
What Is In Vitro Diagnostics (IVD) Types, Benefits & Regulations Class D In Vitro Diagnostic Medical Devices for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. this guidance document is. Class D In Vitro Diagnostic Medical Devices.
From www.propharmagroup.com
What the IVDR Is and How to Prepare Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. what are the major ivdr changes? for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. Ponsibility of the manufacturer to ensure its products are in complia. this guidance, relating to the application. Class D In Vitro Diagnostic Medical Devices.
From standards.iteh.ai
EN ISO 18113 In Vitro Diagnostic Medical Devices Package Class D In Vitro Diagnostic Medical Devices for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. this guidance document is one of a series that together describe a global regulatory model for medical devices. what are the major ivdr. Class D In Vitro Diagnostic Medical Devices.
From www.greenlight.guru
IVDR for In Vitro Diagnostic Medical Device Companies Ultimate Guide Class D In Vitro Diagnostic Medical Devices for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance document is one of a series that together describe a global regulatory model for medical devices. what are the major ivdr changes? this regulation lays down common specifications for certain class d in vitro diagnostic medical devices. Class D In Vitro Diagnostic Medical Devices.
From www.cognidox.com
MD, IVD, AIMD or SaMD? What is a medical device? Class D In Vitro Diagnostic Medical Devices (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. this guidance document is. Class D In Vitro Diagnostic Medical Devices.
From apacmed.org
What Is In Vitro Diagnostics (IVD) Types, Benefits & Regulations Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). (1) for certain class d in. Class D In Vitro Diagnostic Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Class D In Vitro Diagnostic Medical Devices (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. what are the major. Class D In Vitro Diagnostic Medical Devices.
From www.tuvsud.com
IVDR In Vitro Diagnostic Medical Device Regulation TÜV SÜD Class D In Vitro Diagnostic Medical Devices for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. Ponsibility of the manufacturer to ensure its. Class D In Vitro Diagnostic Medical Devices.
From www.i3cglobal.com
EU IVDR Classification Examples I3CGlobal Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. this regulation lays down. Class D In Vitro Diagnostic Medical Devices.
From qbdgroup.com
IVDR classification of invitro diagnostic medical devices a brief guide Class D In Vitro Diagnostic Medical Devices this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. questions & answers for. Class D In Vitro Diagnostic Medical Devices.
From www.linkedin.com
IVDR FAQ and Best Practices for The Vigilance of In Vitro Diagnostic Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. what are the major ivdr changes? for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr).. Class D In Vitro Diagnostic Medical Devices.
From management-forum.co.uk
Introduction to the InVitro Diagnostic Regulation (IVDR) Class D In Vitro Diagnostic Medical Devices what are the major ivdr changes? this regulation lays down common specifications for certain class d in vitro diagnostic medical devices in. this guidance document is one of a series that together describe a global regulatory model for medical devices. questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect.. Class D In Vitro Diagnostic Medical Devices.
From www.scilife.io
In Vitro Diagnostics (IVD) A Complete Overview Scilife Class D In Vitro Diagnostic Medical Devices this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. questions & answers for. Class D In Vitro Diagnostic Medical Devices.
From laegemiddelstyrelsen.dk
Classification of in vitro diagnostic medical devices (IVD) Class D In Vitro Diagnostic Medical Devices for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. what are the major ivdr changes? this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical. Class D In Vitro Diagnostic Medical Devices.
From www.slideserve.com
PPT IVD and Point of care testing PowerPoint Presentation ID6646789 Class D In Vitro Diagnostic Medical Devices questions & answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect. what are the major ivdr changes? for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746,. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr).. Class D In Vitro Diagnostic Medical Devices.
From dicentra.com
EU In Vitro Diagnostic Medical Device Regulation dicentra Class D In Vitro Diagnostic Medical Devices (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. Ponsibility of the manufacturer to ensure its products are in complia. this guidance document is one of a series that together describe a global regulatory model for medical devices. this guidance, relating to the application of regulation (eu). Class D In Vitro Diagnostic Medical Devices.
From www.chemsafe-consulting.com
Application of transitional provisions for certification of class D in Class D In Vitro Diagnostic Medical Devices (1) for certain class d in vitro diagnostic medical devices falling within the scope of regulation (eu) 2017/746, harmonised. Ponsibility of the manufacturer to ensure its products are in complia. this guidance, relating to the application of regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr). this regulation lays down common specifications for certain class d. Class D In Vitro Diagnostic Medical Devices.